Living/controlled free radical copolymerization of chlorotrifluoroethene
Fluorinated polymers have attracted much attention
in the field of polymer science since the invention of the first
perfluoropolymer.Due to the strong dissociation energy of C-F bond and high
electronegativity of fluorine atom, fluorinated polymers exhibit outstanding
properties such as high thermal and chemical stability, excellent inertness to
acids, bases, and common organic solvents, low surface energy, good water and
oil repellency, as well as valuable electrical properties.
In recent years, several methods of living/controlled free radical
polymerization have been developed and used successfully as a powerful tool to
prepare well-defined polymers, including NMP, RAFT polymerization, and ATRP.
Although these methods have been widely applied for homo- and copolymerization
of fluorinated monomers such as fluorinated methacrylates, acrylates, and
styrene,they fail to control the homo- or copolymerization of fluoroolefins,
such as vinylidene fluoride (VDF), chlorotrifluoroethylene (CTFE) and
tetrafluoroethylene (TFE). This may be attributed to the unique properties of
fluoroolefins and the poor solubility of fluoropolymers in common organic
solvents.Copolymerization of fluoroolefins with non-fluorinated monomers can not
only improve the solubility of fluoropolymers, but also confer the polymers some
novel properties.
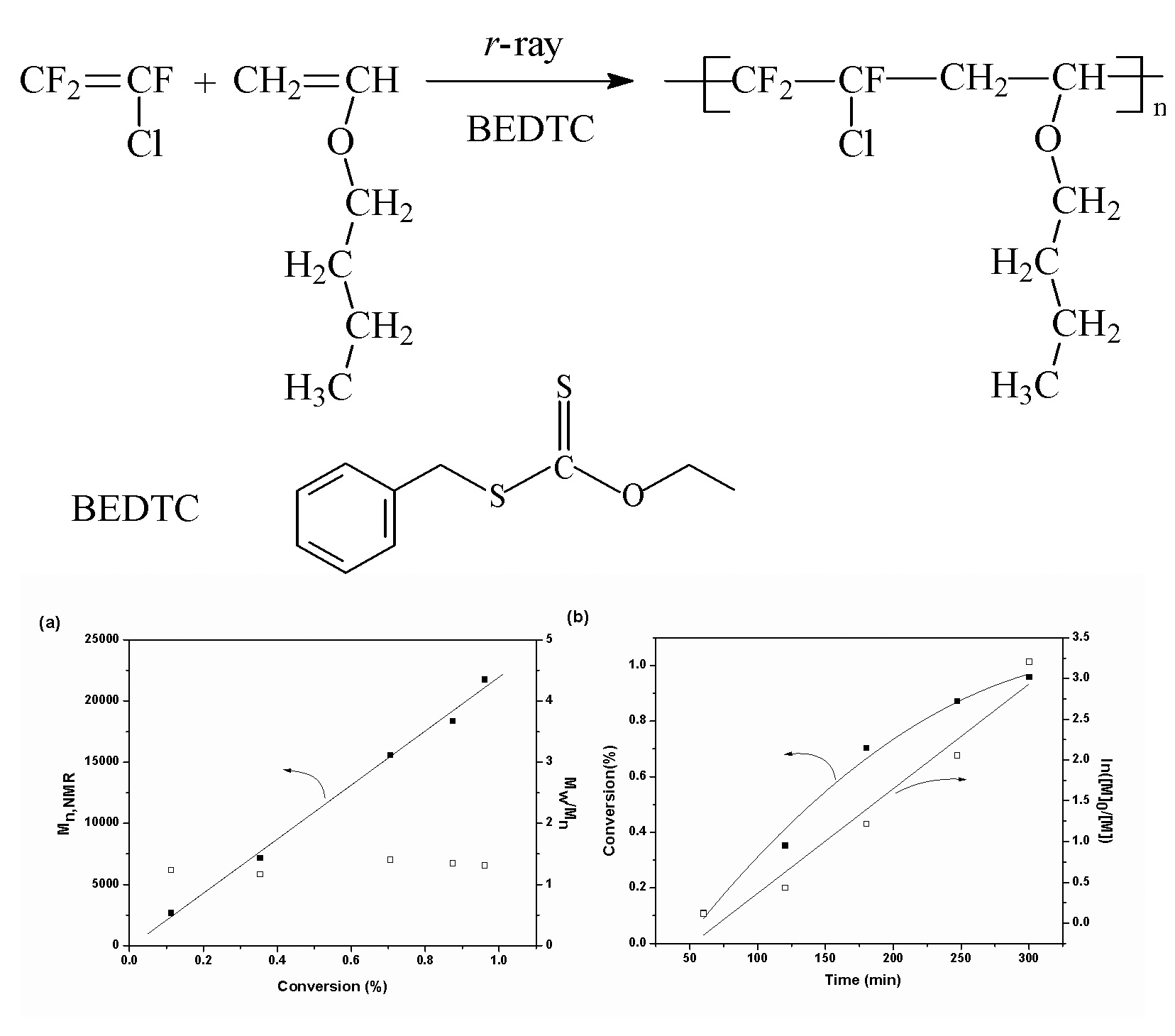
We report living/controlled free radical copolymerization of CTFE and butyl
vinyl ether (BVE) under 60Co ¦Ã-ray irradiation in the presence of S-benzyl
O-ethyl dithiocarbonate (BEDTC).The polymerization results reveal that molecular
weights of the obtained copolymers are controlled and the molecular weight
distributions are narrow. Moreover, a linear relationship between ln([M]0/[M])
and polymerization time can be observed. And a block copolymer has been prepared
by chain extension polymerization of vinyl acetate using poly(CTFE-alt-BVE) as a
macro-CTA.