Overview
Electrochemical process drives energy storage devices such as lithium ion batteries
and supercapacitors. These devices are vital components yet performance limiting factors of portable
electronics, electric vehicles, and smart grids. We are interested in the electrochemistry and the
accompanied structure evolution of the materials. We study the fundamentals of the charge and mass
transport, track the structure change of the materials at different time and length scales, and develop
low cost processings and address critical technique issues toward industrial applications.
Electrochemistry
Electrochemical process determines the upper limit of the electrochemical performance of the
energy storage devices. This process occurs with complex parasitic reactions and materials phase
changes in liquid electrolyte. Such complex process can cover the key information that determines
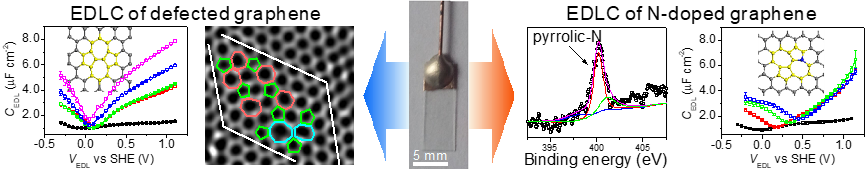
the electrochemical performance. We develop model electrodes of defined structure to provide a neat
environment for to unravel the whole picture that determines the electrochemical process for energy
storage. We invented electrodes consists of graphene sheets prepared by chemical vapor deposition
followed by structure modification. The well-defined electrode structure combines with in-situ
spectroscopy techniques, which allows us to understand in-depth the electrochemical process (Angew
Chem. Int. Ed., 2016, 55, 13822; J. Am. Chem. Soc. 2018, 140, 15568).
Charge and mass transport
Charge and mass transport determine the rate of the electrochemical reactions, thus the power
(charging time) of an energy storage device. The charging time of an electric vehicle is still about
one order of magnitude larger than that to fill up a car with gasoline. Extreme-fast charge requires
materials that allow fast charge and mass transport without the sacrifice of energy. We design
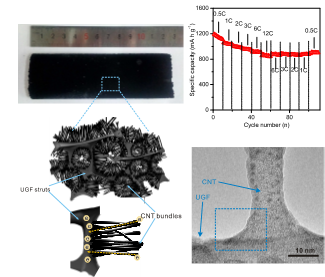
nanostructures and their assemblies to optimize the transport of electron and ions, including their
transference across the phase interface and transport inside the bulk electrode. We invent
covalently connected nanostructures, especially graphitic carbon nanostructures, which accelerate
both the electron and ions transfer of the electrode for applications where extreme-fast charge is
highly demanded (Adv. Mater. 2016, 28, 9094; Adv. Mater. 2017, 29, 1700783; J. Am. Chem. Soc. 2019,
141, 3977).
Carbon materials
Carbon materials, for example graphite, activated carbon, and graphene, are vital components of
the electrodes of electrochemical energy devices. Advanced devices requires these carbon materials
to be achieved with scalable process at low price. We are interested in transferring our knowledge
in the energy storage and carbon materials to the products that are demanded in the market. We
develop synthesis process that can produce carbon materials, for example graphene, with controlled
physical properties at large scale with affordable cost (Natl. Sci. Rev. 2018, 5, 90; Chem. Mater.
2018, 30, 7852).
