|
¾¾ Mechanistic investigation of anticancer metallodrugs
|
|
Tetrathiomolybdate
Induces Dimerization of the Metal-binding Domain of ATPase and Inhibits
Platination of the Protein
|
Nature
Communications,
2019, 10, 186
|
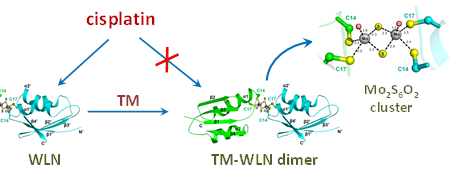
The copper efflux protein ATPase is
associated with the cisplatin resistance. Tetrathiomolybdate (TM) is a drug
used for the treatment of Wilson's disease by targeting ATP7B (WLN). We
found that TM induces dimerization of the metal-binding domain of ATP7B
(WLN4) through a unique sulfur-bridged Mo2S6O2
cluster. The binding of Mo to cysteine residues of WLN4 inhibits platination
of the protein. These results reveal the molecular mechanism how TM
attenuates the cisplatin resistance mediated by copper efflux proteins.
|
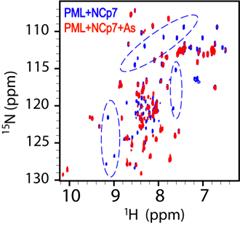
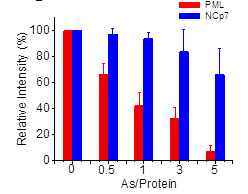
Arsenic
trioxide preferentially binds to the ring finger protein PML: understanding
target selection of the drug
A dual
functional ruthenium arene complex induces differentiation and apoptosis of
acute promyelocytic leukemia cells
|
Chem.
Sci., doi: 10.1039/C9SC03110C
|
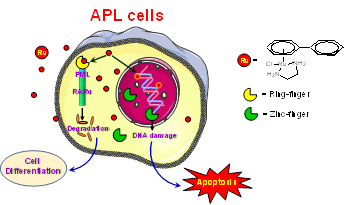
Based on above the
hypothesis, we identified a Ru(II) arene complex, [(¦Ç6-p-bip)Ru(en)Cl][PF6]
(Ru-1) that can selectively react with PML. This reaction leads to
the zinc-release and protein unfolding. Consequently, the degradation of
the fusion protein PML-RAR¦Á occurs, which causes the differentiation of APL
cells. In addition, Ru-1 can also bind to DNA and trigger apoptosis
of APL cells. Therefore, Ru-1 acts as a dual functional agent that
inhibits the growth of APL cells and induces the cell differentiation. On
the contrary, the other non-selective Ru(II) compound, though also highly
reactive to PML, does not exhibit anti-APL activity.
|
Cisplatin
Binds to Human Copper Chaperone Cox17: the Mechanistic Implication of Drug
Delivery to Mitochondria
|
Chem.
Commun. 2014, 50,
2667¨C2669
Biochem.
J., 2015, 472(2)
217¨C223
|
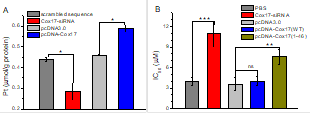
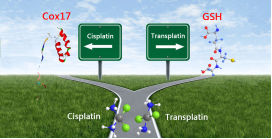
We found that the human copper
chaperone Cox17 facilitates the delivery of cisplatin to mitochondria. The
platinum accumulation in mitochondria is clearly enhanced by the
overexpression of Cox17, and is significantly reduced by silencing the
Cox17 gene. In addition, the expression of Cox17 and its transport to
mitochondria contribute to the cytotoxicity of the drug. In vitro studies
show that cisplatin binds to Cox17 at the copper coordination residues, and
the platination of Cox17 leads to the copper release from the protein.
Although the drug inactive transplatin can also react with Cox17, this
reaction is highly suppressed by glutathione (GSH), the most abundant
cellular reducing agent. On the contrary, the presence of GSH enhances the
reactivity of cisplatin to Cox17. In addition, the pre-formed cisplatin/GSH
adducts are more readily to react with Cox17, and cisplatin can transfer
from glutathione to Cox17. These results indicate that Cox17 could
contribute to the platinum drug transport to mitochondria, and glutathione
plays crucial roles in modulating the reactivity of various platinum
complexes.
|
|
|
¾¾ Design of novel anticancer metallodrugs
|
|
Aspirin
Ligation Enhances Cisplatin Efficacy by Altering the Cellular Response
|
Chem.
Commun., 2014,
50(56),7427¨C7430,
Metallomics, 2016, 8, 672-678
|
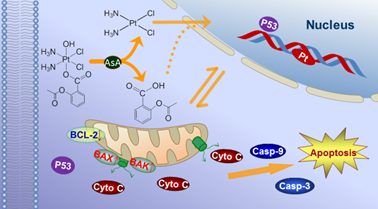
A novel conjugate
of platinum (IV) prodrug has been designed and synthesized by the ligation
of aspirin to cisplatin, to generate c,c,t-[PtCl2(NH3)2(OH)(aspirin)]
(asplatin). Asplatin exhibits
significant cytotoxicity
to tumor cells, up to 10 times more effective than cisplatin. In addition,
asplatin almost fully overcomes the drug resistance of the cisplatin
resistant cells. Asplatin is highly accumulated in cancer cells; upon the
reduction with cellular ascorbic acid, asplatin is activated and binds to
DNA more efficiently than cisplatin. Meanwhile, the aspirin released from
asplatin also modulate the cellular response to the platinum agent.
Mechanistic studies reveal that the aspirin ligand promotes the apoptosis
via the BCL-2 associated mitochondrial pathway, which greatly sensitizes
the tumor cells to the cisplatin converted from asplatin. Therefore, the
ligation of aspirin to the platinum drug exhibits the great synergistic
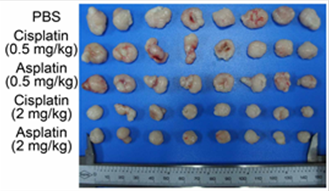
effect in the low micromolar range. The in vivo experiment shows
that asplatin exerts a significant inhibitory effect on tumor growth with
lower systemic toxicity compared with cisplatin. This result offers a novel
strategy to enhance sensitivity of platinum drugs by ligation of the anti-inflammation
drug aspirin. [Highlighted in Chemistry World]
|
|
|
¾¾ Drug delivery
|
|
Modular
design of nanobody-drug conjugates for targeted-delivery of platinum
anticancer drugs with an MRI contrast agent
|
Chem.
Commun., 2019, 55,
5175-5178
|
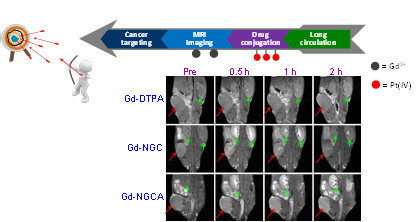
Targeted delivery is highly desirable
in cancer chemotherapy, particularly for cisplatin. We developed a
multifunctional nanobody-drug conjugate (NDC) for targeted delivery of
platinum(IV) prodrug and MRI contrast agent. NDC can be specifically
internalized into EGFR positive cancer cells. Therefore, the drug
accumulation is increased in EGFR positive tumor and decreased in major
orangs, resulting in higher therapeutic effect and lower side-effects in
comparison to the treatment of cisplatin. The fusion of anti-albumin
nanobody improves the pharmacokinetic properties of NDC, which further
enhances the drug efficacy. In addition, the Gd-binding domain enables in
situ detection of the drug distribution in vivo.
|
Oral
Delivery of Platinum Anticancer Drug Using Lipid Assisted Polymeric
Nanoparticles
|
Chem.
Commun., 2015, 51,
17536¨C17539
|
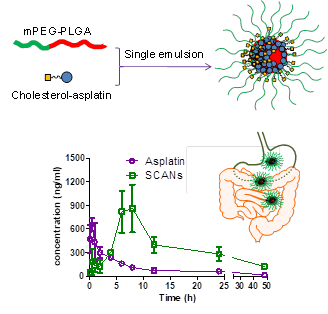
Oral administration is highly desirable
in chemotherapy, particularly for drugs with severe side-effects, eg.
platinum anticancer drugs. Although Pt(II) antitumor drugs can be treated
only via intravenous injection, Pt(IV) complexes have shown high oral bioavailability.
However, the undesired reduction limits the effectiveness of Pt(IV) agents.
Here, we present a nano-delivery platform for the oral administration of
Pt(IV) prodrug using the lipid assisted polymeric nanoparticles. The self-assembled
cholesterol-asplatin-incorporated nanoparticles (SCANs) exhibit enhanced
cellular uptake and sustained drug release. SCANs exhibit higher inhibitory
efficiency compared with free cisplatin on multiple cancer cells, including
cisplatin resistant cells. Pharmacokinetic study reveals that SCANs
demonstrate significantly improved oral bioavailability with the prolonged
drug release time and the postponed drug clearance in comparison to free
Pt(IV) prodrugs. In vivo assay shows that the oral administration of SCANs
effectively inhibits the tumor growth with significantly lower nephrotoxicity
and systemic toxicity compared with intravenous treatment of cisplatin. [Highlighted in Chemistry World]
|
Charge-Selective
Delivery of Proteins Using Mesoporous Silica Nanoparticles Fused with Lipid
Bilayers
|
ACS
Appl. Mater. Interfaces, 2019, 11, 3645-3653
|
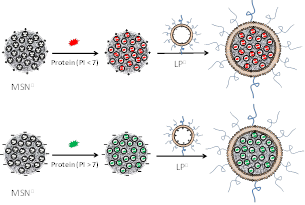
We developed a platform for efficient
protein delivery using mesoporous silica nanoparticles (MSN㊉ and MSN㊀). The cargo proteins, based on the
surface charges, can be selectively loaded in very high efficiency. The
lipid fusion significantly increases the stability of the nano-system in
physiological condition, and the MSN-LP protocell can efficiently deliver
proteins into cells. Proteins can maintain their functions after delivery
into cells.
|
Human
Serum Albumin Conjugated Nanoparticles for pH and Redox-Responsive Delivery
of a Prodrug of Cisplatin
|

Chem.
Eur. J. 2015,
21(46), 16547¨C16554
|
A highly biocompatible, pH and redox
dual-responsive delivery system is prepared using the hybrid nanoparticles
of human serum albumin (HSA) and calcium phosphate (CaP) for the Pt(IV)
prodrug of cisplatin. This conjugate is very stable under the extracellular
conditions, so that it protects the Pt(IV) prodrug in HSA. Upon reaching
the acidic and hypoxia environment, the platinum drug is released in its
active form and is able to bind to the target DNA. Interestingly, this
delivery system demonstrates enhanced cytotoxicity to tumor cells, but not
to normal cells.
|
|
|
¾¾ Mechanistic investigation of metalloproteins
|
|
Cuprous
binding promotes interaction of copper transport protein hCTR1 with cell
membranes
|
Chem.
Commun., 2019, 55,
11107-11110
|
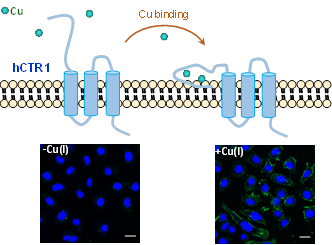
Human copper transporter (hCTR1) is a
plasma membrane protein that facilitates the cellular uptake of cuprous
ions. The extracellular N-terminal metal binding domain (MBD) of hCTR1 is
proposed to capture copper ions and transfer them into cells via
coordination transfer. We found that Cu(I) ions bind to the N-terminal MBD
of hCTR1 to form a Cu(His)2(Met)2 coordination and
induce its conformational change. This alteration promotes the interaction
of N-terminal MBD with cell membranes, which is confirmed on DPPC liposomes
and on living cells as well. Confocal fluorescence images clearly showed
that, upon Cu(I) coordination, hCTR11-46 binds to the membrane
of cells. These results reveal that copper binding triggers the
conformation change of N-terminal MBD of hCTR1 and leads to the membrane
interaction, which can be a crucial step to initiate the cellular uptake of
copper ions by hCTR1.
|
Copper-Finger
Protein of Sp1: the Molecular Basis of Copper Sensing
|
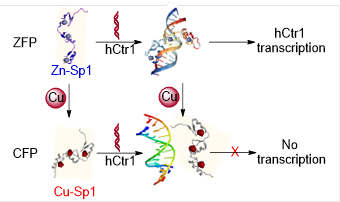
Metallomics, 2017, 9, 1169-1175
|
The expression of copper transport protein
hCtr1 is transcriptionally regulated by specifity protein 1 (Sp1) in
response to the cellular copper level. We found that Sp1 demonstrates high
binding affinity to cuprous ions, even stronger than Cu-Atox1 binding.
Cu(I) can displace Zn(II) in Sp1, resulting in a well-folded
¡®Copper-Finger-Protein¡¯ (CFP). Although only very little structural alteration occurs upon the copper
binding, CFP cannot recognize
the promoter of hCtr1, therefore copper binding interrupts the transcription. This result indicates that, in addition
to apo-to-holo alteration, the metal substitution can also lead to the
transcriptional switch in metal sensing.
|
|
|
|
|
Back Home
|
|
|