Solid–liquid phase transition induced electrocatalytic switching from hydrogen evolution to highly selective CO2 reduction
Published in Nature Catalysis, March 11, 2021
Hongfei Liu#; Jun Xia#; Nan Zhang; Han Cheng; Wentuan Bi; Xiaolong Zu; Wangsheng Chu; HengAn Wu; Changzheng Wu*; Yi Xie; Solid–liquid phase transition induced electrocatalytic switching from hydrogen evolution to highly selective CO2 reduction, Nature Catalysis, 2021, 4(3): 202–211. https://doi.org/10.1038/s41929-021-00576-3
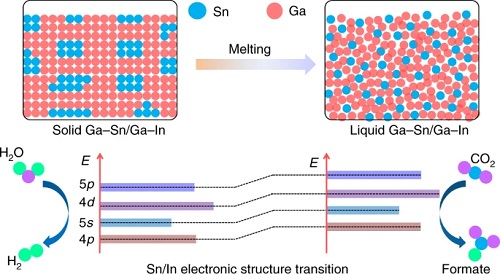
Abstract: Conventional strategies for modifying electrocatalysts for efficient CO2 reduction are mainly based on doping, defect/morphology engineering, substrate design and so on. In most cases, these methods can only tune their structures, electronic states and thereby catalytic properties in a gradual way. Here we report that the solid–liquid phase transition of Ga–Sn/Ga–In alloys can induce an instant and radical transformation of their atomic and electronic structures during electrocatalysis, which dramatically impacts their catalytic properties. The transition of Sn/In active components from phase-segregated clusters to dispersed single atoms during melting results in a unique electronic structure through further reduction of both metallic Sn/In and Ga. Such atomic/electronic structure transitions can correlate well with suppression of the hydrogen evolution reaction and an enhanced formate Faradaic efficiency from <35% to >95%. This two-state switching strategy may be extended to other catalytic reactions to determine correlations between their structures and catalytic properties.
