
Major reaction pathways for tcy-C6H10(∗)CH3 + O2 at the QCISD(T)/CBS//B3LYP/6-311 ++ G(d,p) level.

Comparison between our best prediction and previously reported rate constants for phenyl + O 2 recombination.
Related articles:
Recombination of aromatic radicals with molecular oxygen.
DOI: 10.1016/j.proci.2016.06.021
Theoretical kinetic studies for low temperature oxidation of two typical methylcyclohexyl radicals.
DOI: 10.1016/j.combustflame.2017.03.025
2. Crucial conjugated intermediates and their contribution to the formation of aromatic compounds.
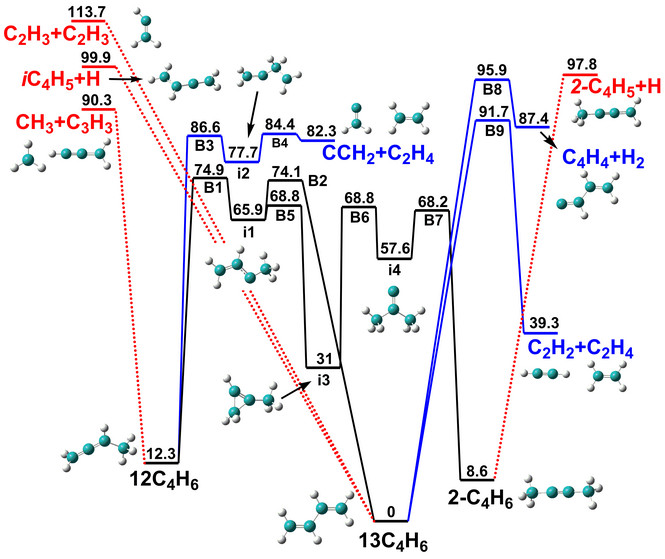

Related articles:
Initiation mechanism of 1,3-butadiene combustion and its effect on soot precursors.
DOI: 10.1016/j.combustflame.2017.06.003
Pressure-dependent kinetics on the C4H7 potential energy surface and its effect on combustion model predictions.
DOI: 10.1016/j.combustflame.2017.01.031
3. OH-initiated
low-temperature oxidation of VOCs in atmosphere -- similarity and
difference between combustion chemistry and atmospheric chemistry.
Related articles:
Pressure-Dependent Kinetics of the Reaction between CH3OO and OH Focusing on the Product Yield of Methyltrioxide (CH3OOOH)
DOI:pubs.acs.org/doi/10.1021/acs.jpclett.9b00781

