Research
Main content
To better understand, describe, optimize and predict the behaviors in complex chemical experiments,
multiscale modeling becomes a useful methodology and has been widely applied it to deal with chemical systems.
Recently, extensive studies of chemical systems have been downscaled from macro to micro scale such as nanocatalysis,
growth of two-dimensional materials and self-assembly of nanomaterials. Statistical methods will help us understanding and optimizing these systems.
We have built multiscale statistic-dynamical model from single atoms to large scale cluster for the promising cognition of these systems,
such as epitaxial growth of two dimensional materials, assembly of nanowires, modeling of electrocatalysis.
Representative results are given as follows:
Multiscale Modeling of Nonlinear Epitaxial Growth of Graphene
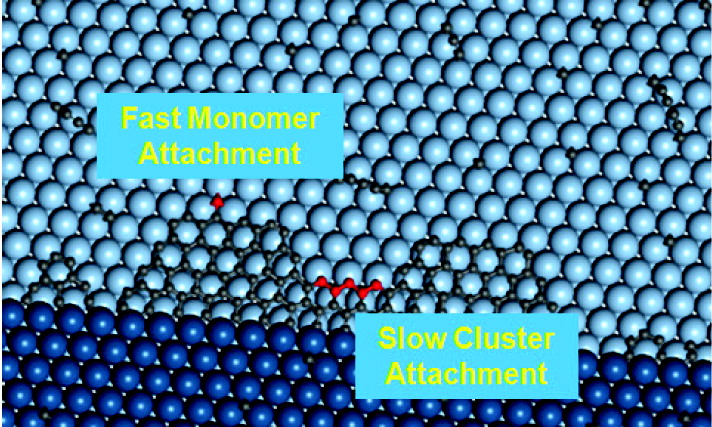
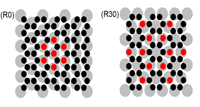
Graphene has attracted intense research interest due to its unique electronic structure and great application potential whose epitaxial growth on metal surfaces can generate a large graphene sample with high quality. To understand the atomic mechanism of a rather counterintuitive phenomenon and bridge the huge time scale separation between atomic processes and the experimental kinetics, we have developed a multiscale approach combining DFT calculations with a standing-on-front kinetic Monte Carlo simulation scheme.
Detailed analysis shows that lattice mismatch between the graphene and metal substrate is the very reason for the nonlinear growth behavior (J. Am. Chem. Soc. 2012). In addition, the growth exponent is shown to be orientation sensitive, and it is geometrically determined by the Morie pattern between graphene and the substrate (Phys. Rev. B 2013).
J. Am. Chem. Soc. 2012, 134, 6045
Kinetics of Tip/Roughness-Enhanced Selectivity of Electrocatalytic Reduction
Although many assembly strategies have been used to successfully construct well-aligned nanowire assemblies,the understanding of their assembly kinetics has remained elusive, which restricts the development of NWbased device and circuit fabrication. A versatile strategy is presented to track the assembly evolution of the NWs in real time. During the interface assembly process, the randomly dispersed NWs gradually aggregate to form small ordered NWblocks and finally are constructed into well-defined NW monolayer driven by the conformation entropy.
The NW assembly mechanism can be well revealed by the thermodynamic analysis and large-scale molecular dynamics theoretical evaluation. These findings point to new opportunities for understanding NW assembly kinetics and manipulating NW assembled structures by bottom-up strategy.
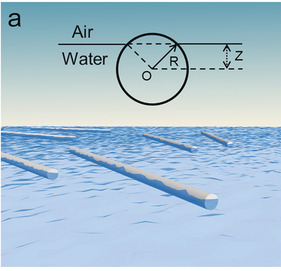
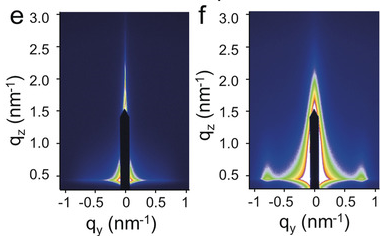
Angew.Chem.Int. Ed. 2018, 57,8130–8134
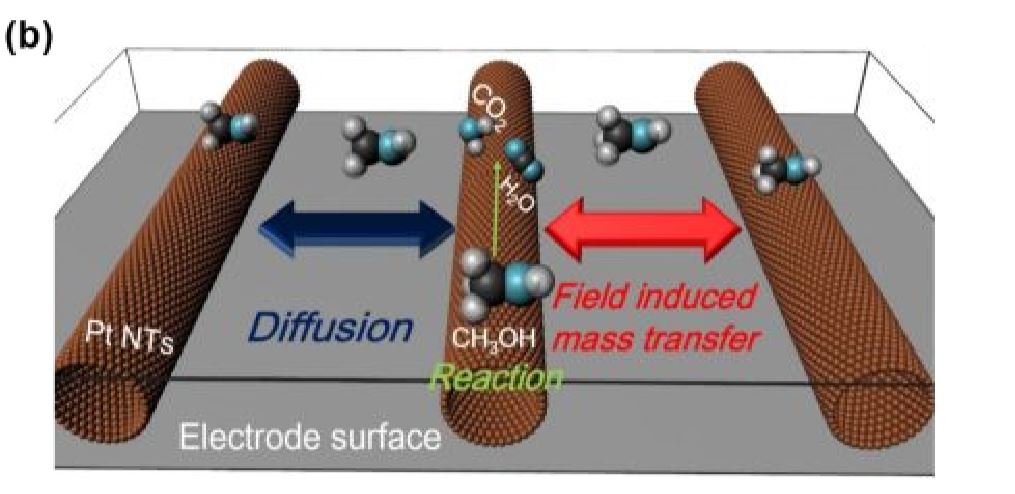
High roughness has been proved to be an effective design strategy for electrocatalyst in many systems. Especially, high selectivity of carbon monoxide reduction (CORR) in competition with the hydrogen evolution reaction has been observed on high roughness electrocatalysts. However, the two well-known mechanisms, i.e., decreasing the energy barrier of CORR and increasing local pH, failed to understand the roughness-enhanced selectivity in a recent experiment. We unraveled the hidden mechanism by establishing a comprehensive kinetic model for CORR on catalysts with different roughness factors. We conclude that the roughness-enhanced CORR selectivity is actually kinetic controlled by local electric-field-directed mass transfer of adsorbed species on the electrode surface.
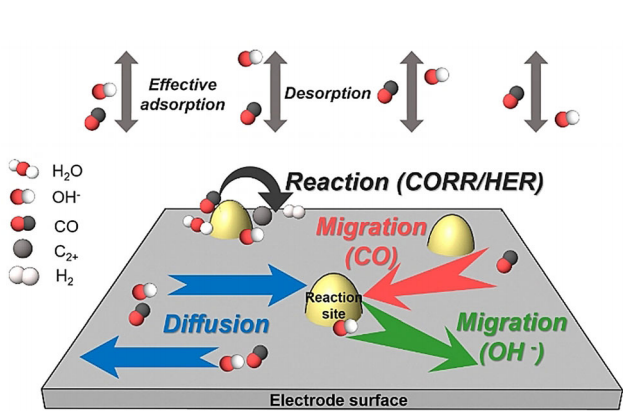
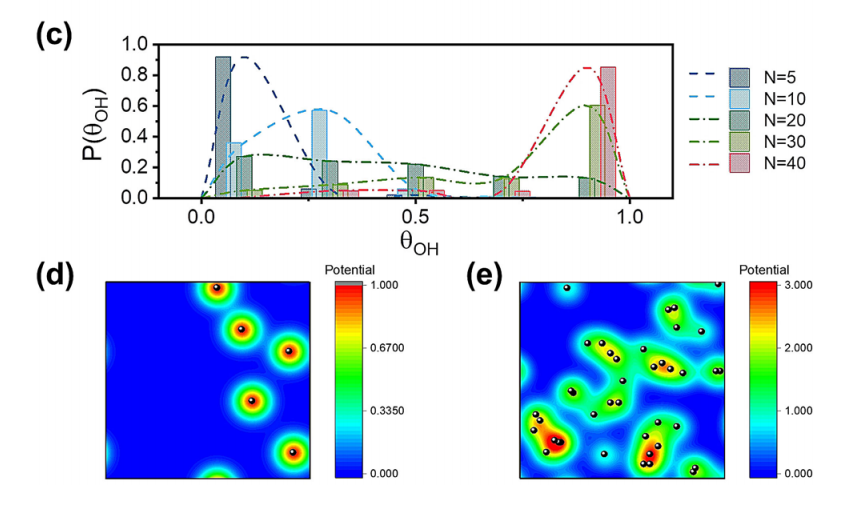
Angew.Chem.Int. Ed. 2020, 60,1-6
Ordered Nanostructure Enhances Electrocatalytic Performance by Directional Micro-Electric Field
Great achievements have been made to optimize thermodynamics to reduce energetic barriers of the catalytic reactions. However, little attention has been paid to design catalysts to improve kinetics to enrich the local concentration of reactant molecules surrounding electrocatalysts. Here, we find that well-designed nanocatalysts with periodic structures can optimize kinetics to accelerate mass-transport from bulk electrolyte to the catalyst surface, leading to the enhanced catalytic performance.
This achievement stems from regulation of the surface reactant flux due to the gradient of the microelectric field directing uniformly to the nearest catalyst on ordered pattern, so that all of the reactant molecules are utilized sufficiently for reactions, enabling the boost of the electrocatalytic performance. This novel concept is further confirmed in various catalytic systems and nanoassemblies, such as nanoparticles, nanorods, and nanoflakes.
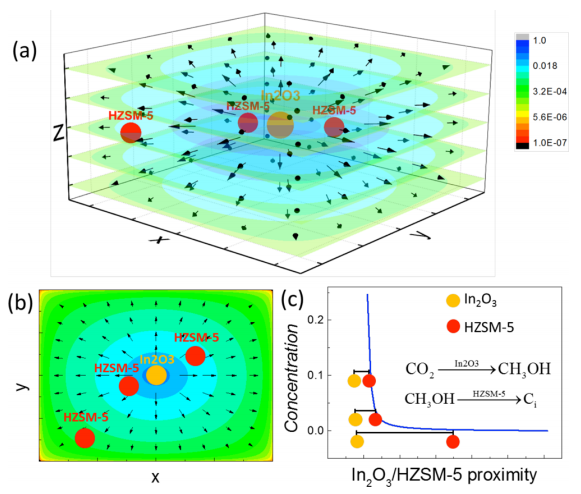
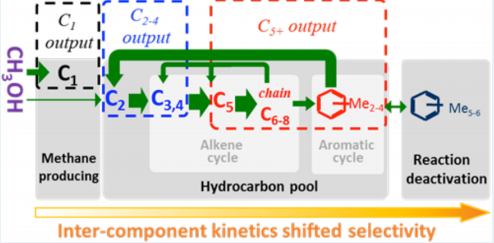
Proximity-Dependent Selectivity of Carbon Dioxide Reduction on Bifunctional Catalysts
We reveal the well-known proximity-dependent selectivity Multifunctional catalysts is closely associated with the kinetics involved. Based on reaction−diffusion dynamics together with kinetic Monte Carlo simulation on a coarse-grained model, we found that the diffusion kinetics of the intermediate methanol generated on In2O3 plays a decisive role for the selectivity. For different In2O3/HZSM-5 proximities, the local methanol concentration induces a shift of the dominant process for subsequent methanol-to- hydrocarbon reactions inside HZSM-5, resulting in a preferred reaction window to generate favorable liquid fuels with profound high selectivity.